
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
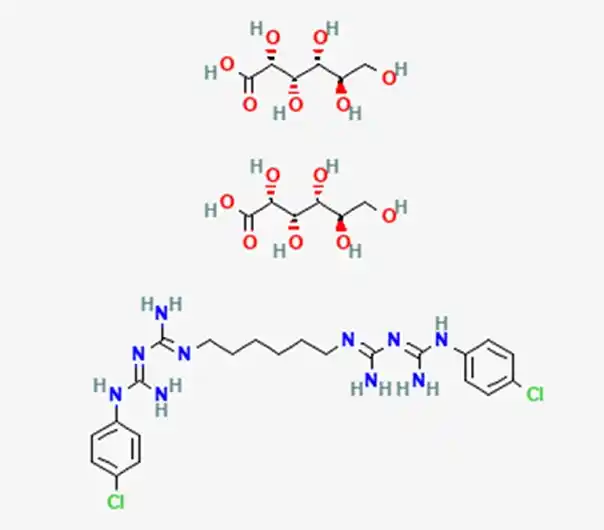
C34H54Cl2N10O14
(1E)-2-[6-[[amino-[(E)-[amino-(4-chloroanilino)methylidene]amino]methylidene]amino]hexyl]-1-[amino-(4-chloroanilino)methylidene]guanidine;(2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoic acid
18472-51-0
897.76 g/mol
Chlorhexidine
Antiseptic
| Appearance | White to off-white crystalline powder. |
|---|---|
| Solubility | Soluble in water, particularly in its digluconate salt form |
| Melting point | 134 to 136°C |
| pH | 134 to 136°C |
Chlorhexidine gluconate is a disinfectant and antiseptic agent, commonly used to reduce bacteria in various settings. It's found in oral rinses for gingivitis treatment and as a skin disinfectant before surgeries or procedures involving catheters. It works by disrupting bacterial cell membranes, leading to cell death.
Chlorhexidine gluconate is a broad-spectrum antiseptic that works by binding to negatively charged bacterial cell walls, disrupting membrane integrity. At low concentrations, it is bacteriostatic, causing leakage of intracellular components, while at higher concentrations, it is bactericidal, leading to cell lysis. It is effective mainly against Gram-positive and some Gram-negative bacteria, fungi, and enveloped viruses.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Chlorhexidine gluconate is widely used in infection prevention, particularly in surgery, dentistry, wound care, and hospital hygiene. It is prescribed for preoperative skin disinfection, oral care (e.g., gingivitis, plaque control), and cleansing of wounds and catheter sites.
Salius Pharma facilitates market entry by providing DMFs, Certificates of Analysis (CoAs), and CTD/eCTD dossiers tailored to meet the regulatory requirements of authorities such as ISP (Chile), Digemid (Peru), and ANMAT (Argentina), ensuring smooth compliance for pharmaceutical formulators and distributors.
Salius Pharma supplies a complete package including GMP certificates, batch-specific CoAs, MSDS, stability data, and shipping compliance forms, ensuring hassle-free customs clearance, regulatory compliance, and reliable supply for local manufacturers in LATAM markets.
Prolonged use may cause skin or mucosal irritation, tooth staining (oral use), or hypersensitivity reactions. Rarely, serious allergic reactions (e.g., anaphylaxis) can occur. It should not be used in the eyes, ears (if perforated), or deep wounds.
Chlorhexidine gluconate disrupts bacterial cell membranes by binding to negatively charged sites, increasing permeability, and causing leakage of cellular contents. It is bacteriostatic at low concentrations and bactericidal at higher concentrations.
Common side effects include skin irritation, oral mucosal burning, tooth staining, and taste disturbances. Rare side effects include allergic reactions and anaphylaxis.
Looking to source Chlorhexidine Gluconate or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.